MITRA-FR vs. COAPT: Conflicting Results Harmonize to Deduce Ideal HF-FMR Patient for MitraClip
[AP VALVES & SH 2021] Insights gleaned from opposing MitraClip results help define optimal anatomical features for TEER
Analysis of two MitraClip studies with seemingly contradictory results is helping interventional cardiologists chisel a more detailed profile of the optimal candidate for transcatheter edge-to-edge mitral valve repair (TEER) in the realm of heart failure (HF).
Results from the COAPT and MITRA-FR studies - both published in 2018 - fueled a heated debate on which patient groups benefit from the transcatheter procedure, mainly since the two randomized controlled trials (RCT) studied the same MitraClip (Abbott Vascular) device and technique on virtually the same HF patient group with secondary mitral regurgitation (MR), but produced antipodal results.
 Susheel Kodali, MD (New York-Presbyterian Columbia University Irving Medical Center, New York, USA) and panelists at AP Valves & Structural Heart Virtual (AP VALVES & SH 2021) on Aug. 6 discussed in-depth where exactly MitraClip fits on the spectrum of evolving HF therapies.
Susheel Kodali, MD (New York-Presbyterian Columbia University Irving Medical Center, New York, USA) and panelists at AP Valves & Structural Heart Virtual (AP VALVES & SH 2021) on Aug. 6 discussed in-depth where exactly MitraClip fits on the spectrum of evolving HF therapies.
"We have to ask ourselves whether TEER with MitraClip can interrupt or slow progression of a vicious cycle wherein volume overload to the left ventricle (LV) leads to functional mitral regurgitation (FMR), which increases volume overload and dilation for worsening HF, and so forth," Kodali said.
"HF has changed over a decade. It was initially just diuretics, then came medications like neuro-hormonals, beta-blockers, ACE inhibitors, etc. Functional status improved with all these therapies, and the improvement - at times - hold for years; however, the question is when functional status decompensates, where does MitraClip fit on the spectrum of guideline-directed medical therapy (GDMT), and LVADs and transplants?" he asked.
Through COAPT and MITRA-FR analysis, Kodali drew up a rough answer that pointed to specific patient characteristics that included:
- Continuing HF symptoms despite maximally-tolerated GDMT
- Favorable anatomies: lack of calcium, clefts, broad jets, and small mitral valve anatomy (MVA)
- Disproportionate MR
"If we sum up the lessons learned, treatment with MitraClip in patients with worsening heart failure despite GDMT does result in a sustained clinical benefit that translates to benefit in quality of life. Patients who received TEER later, as well as patients with pulmonary hypertension or residual gradient, also benefitted," he said. "But we need future studies to determine whether indications should be broadened to populations such as those with anatomic restrictions or earlier treatment."
Also doubling as a speaker and panel discussant at AP VALVES & SH 2021, Saibal Kar, MD (Los Robles Regional Medical Center, California, USA) set clear boundaries for TEER: "MitraClip is reasonable in intermediate-risk patients but not high-risk. This is true at least in the US where trials comparing the clip versus surgery for intermediate-risk show that it is reasonable."
"Having said that, I believe there are some anatomies that are better with surgery, such as anterior prolapse," he added. Although he reaffirmed MitraClip for intermediate-risk patients, he also stated that he has problems with low-risk TEER and is "not ready for that."
Piecing together the MitraClip puzzle for HF with secondary MR
The radically different results between the two studies - COAPT being positive and MITRA-FR being neutral - have spurred much data sifting to pan out the golden TEER candidate in clinical practice.
Cardiologists handling HF must sometimes make treatment decisions without all the evidence in place for specific patient groups; this ambiguous grey zone also extends to the non-surgical treatment of HF with secondary MR.
The challenge is to go forward rather than stay behind and wait too long with the patient failing multiple rounds of medical therapy.
MR is a condition where the mitral valve does not correctly coapt - or gather closely together for closure - and causes blood to flow backward into the heart instead of out to the body. Severe MR can block blood flow to the rest of the body, leading to worsening HF symptoms.
But MR is "not a single disease entity," Kodali pointed out. It can be classified as either primary MR caused by disease in the mitral leaflets, or secondary MR (also called functional MR) due to coronary disease in the left ventricle (LV) or left atrium (LA), not the mitral valve leaflets.
Secondary to surgical repair, TEER with the small metal "clipping" device known as the MitraClip is becoming an increasingly popular transcatheter treatment for secondary MR, in addition to primary MR.
MitraClip gained initial U.S. Food and Drug Administration (FDA) approval in 2013 for patients with primary MR and at high risk for mitral valve surgery.
The FDA further expanded indications for MitraClip on March 2019 to patients with HF symptoms with moderate-to-severe or severe secondary MR despite maximally-tolerated GDMT1.
MitraClip expectations take rollercoaster ride with trial results
Before the much compared and contrasted MITRA-FR and COAPT studies, findings from the Abbott Vascular-funded EVEREST II trial published in the New England Journal of Medicine (NEJM) in 2011 showed superior safety and similar outcome improvements with MitraClip compared to surgery in high-risk HF patients, and subsequently increased its hype.2
But in August 2018, Jean-François Obadia, MD (Civils Hospices of Lyon, Lyon, France) and colleagues published the MITRA-FR (Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation)3 results in NEJM that showed no outcome difference between MitraClip and GDMT versus GDMT alone - dampening the once-high expectations.
The prospective RCT - funded by the French Ministry of Health and Research National Program and Abbott Vascular - included 304 HF patients with severe secondary MR (defined as an effective regurgitant orifice area (EROA) �� 20 mm2 or regurgitant volume (RV) >30 mL/beat) and LVEF between 15-40% to examine the rate of death or unplanned hospitalization for HF at 1-year.
MITRA-FR results showed MitraClip had no benefit over drug therapy alone (OR 1.16, 95% CI 0.73-1.84, p=0.53). Death from any cause (HR 1.11, 95% CI 0.69-1.77) and hospitalization rate (HR 1.13, 95% CI 0.81-1.56) between the two groups also failed to prove significant difference.
On the heels of the MITRA-FR publication, however, came findings from the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation)4 trial by Gregg W. Stone, MD (Mount Sinai Icahn School of Medicine, New York, USA) and colleagues in the NEJM in September.
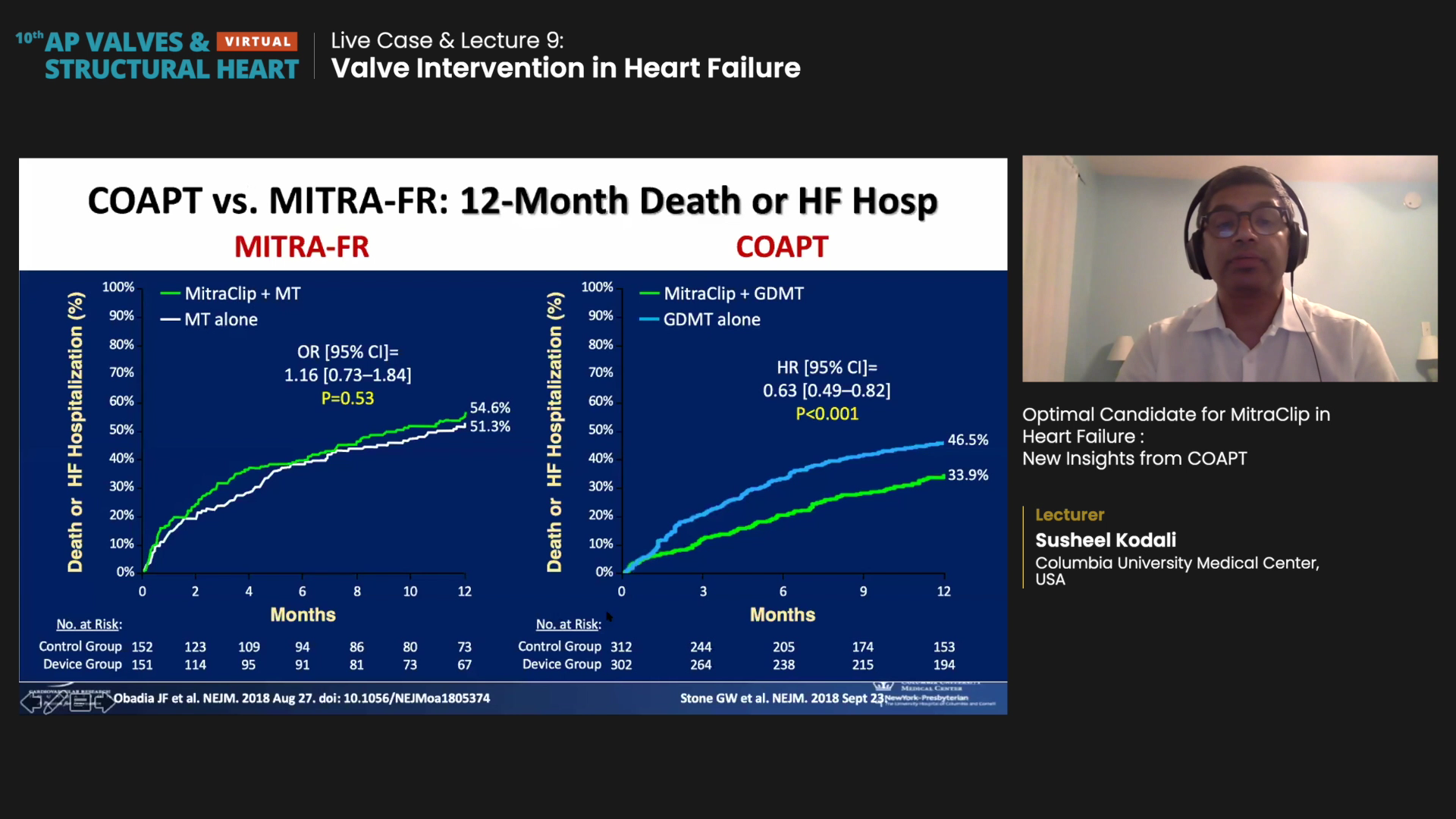
The COAPT study, once again funded by Abbott, showed that MitraClip slashed risks of HF-related hospitalization and all-cause mortality at 2-years compared to GDMT alone.
The multicenter, parallel-controlled, open-label COAPT trial included 614 HF patients from about 80 U.S. and Canadian centers with moderate-to-severe (3+) or severe (4+) secondary MR who remained symptomatic despite maximally tolerated GDMT to either MitraClip with GDMT (n=302) or GDMT alone (n=312).
"Dramatic" results at 24 months of follow-up showed the annualized rate of all-hospitalizations for HF was 35.8% per patient-year in the MitraClip group and 67.9% per patient-year in the control group, thereby halving the risk of hospitalization (HR 0.53, 95% CI, 0.40-0.70, p<0.001).
MitraClip also lowered risk of death at 24 months from any cause by 38% (Mitra 29.1% vs. GDMT 46.1%, HR 0.62, 95% CI, 0.46-0.82, p<0.001).
"The dramatic results from COAPT were, to be honest, a bit unexpected. The outcome curves [between the device and control group] start to separate at 1-year, but you don't see this separation in MITRA-FR," Kodali said. "Positive MitraClip outcomes came despite being performed on patients taking HFrEF therapies - and these therapies (ACE inhibitors, sacubitril/valsartan, beta-blockers, etc.) have been proven to reduce mortality."
These long-awaited results inevitably stirred much discussion among cardiologists and cross-examination of trial outcomes. Eyes are now set on the upcoming but ongoing RESHAPE-HF2 trial5.
Different results chalked up to different enrollment criteria of secondary MR
Comparing these two trials, Kodali explained that the two studies actually had different patient populations.
For starters, MITRA-FR had a smaller population (n=304) than COAPT (n=614).
Also, entry criteria for severe MR were different. MITRA-FR used European guidelines (EROA >20mm2 or RV >30 mL/beat) while COAPT used the more stringent American guidelines (EROA >30mm2 or RV >45 mL/beat).
As a result, MITRA-FR had a mean EROA of 31 ��10 mm2 while COAPT had a mean EROA of 41 ��15 mm2. Mean left ventricular end-diastolic volume (LVEDV) was also larger in MITRA-FR (135 ��35 mL/m2 vs. 101 ��34 mL/m2), indicating patients with larger LVs and less MR.
"We have to carefully consider the range and spectrum of LV dysfunction as two patients with the same EROA of 30mm2 may benefit from different therapies," Kodali said. "For instance, two patients with an EROA of 30 mm2 but differing LVEF and LV size/geometry (normal to severely abnormal) will require different treatment ranging from MR correction to LVAD, transplant, or hospice."
Patients in MITRA-FR were also more aligned with non-severe MR, while COAPT had more patients with disproportionally severe MR.
"The outcomes depended on the procedural result. If you had residual MR (3+ or 4+), the outcomes were not great and looked more like GDMT. But if you had less than 2+ MR, those were the patients that did well," Kodali said.
Later treatment with MitraClip also had comparable outcomes as early (initial) treatment.
"We can learn something from the crossovers as well. After the 2-year endpoint, 58 patients were allowed to crossover to the MitraClip procedure. Acute results were good with 2+ MR for 96% of patients, even though it had been two years since they were enrolled," he said. "Even later treatment translated into similar clinical benefit, indicating we should focus on earlier treatment."
"The challenge is to go forward rather than stay behind and wait too long, with the patient failing multiple rounds of medical therapy," Kodali added.
Saibal added to the discussion, "Overall, case selection and attention to detail during the procedure are critical for the procedure's success. Case selection refers to etiology, MR severity, surgical risk, and morphological criteria. Most importantly, you need to know when to stop and which cases to avoid."
During his presentation, Saibal emphasized that operators should avoid using MitraClip in patients with mitral valve orifice less than 3.5 cm2; rheumatic MR with commissural fusion; calcified leaflets; severe TR that cannot be treated; recent endocarditis; or MR due to congenital cleft (endocardial cushion defects).
Having a good imager is also essential, Saibal said, noting that imagers are just as important as the interventional cardiologists - if not more.

